
Blood cancer patients in England are set to be among the first in the world to access a pioneering “Trojan horse” treatment, health officials have announced.
The targeted therapy, belantamab mafodotin, also known as Blenrep, has shown promising results in halting the progression of myeloma for nearly three times as long as current treatments, according to studies.
Approximately 1,500 patients annually with multiple myeloma, an incurable bone marrow cancer, are expected to benefit from this new treatment.
The National Institute for Health and Care Excellence (Nice) has approved Blenrep, manufactured by GlaxoSmithKline, for use within the NHS.
NHS England has confirmed that it will be the first health system worldwide to roll out the treatment.
The drug will be available to patients whose cancer has advanced or has not responded to initial treatments. Administered as an infusion every three weeks alongside other cancer drugs, this antibody drug targets and attaches to cancer cells.
It has been dubbed a “Trojan horse” treatment because it works by being taken into a cancer cell, before releasing a high concentration of a lethal molecule to destroy the cell from inside.
“Myeloma is an aggressive type of blood cancer, but we have seen a steady improvement in the outlook for patients over recent years as we have introduced new targeted therapies,” Professor Peter Johnson, NHS England’s national clinical director for cancer, said.
“I am delighted that patients in England will be the first to benefit from this new treatment, which has the potential to keep cancer at bay for years longer, giving people the chance of more precious time with friends and family.
“This treatment could be life-changing for many patients and their families, and that’s why it is so important that the NHS continues to secure quick access to the latest, innovative treatments like this, at affordable prices to the taxpayer.”
Helen Knight, director of medicines evaluation at Nice, said: ”We’re delighted that people in the UK will become among the first in the world to access belantamab mafodotin for this indication.
“This recommendation demonstrates our commitment to getting the best care to patients fast, while ensuring value for the taxpayer.”
Trials have suggested that the treatment, in combination with bortezomib and dexamethasone, delayed progression of the disease by an average of three years, compared to just over a year for patients taking commonly-used drug daratumumab along with the other treatments.
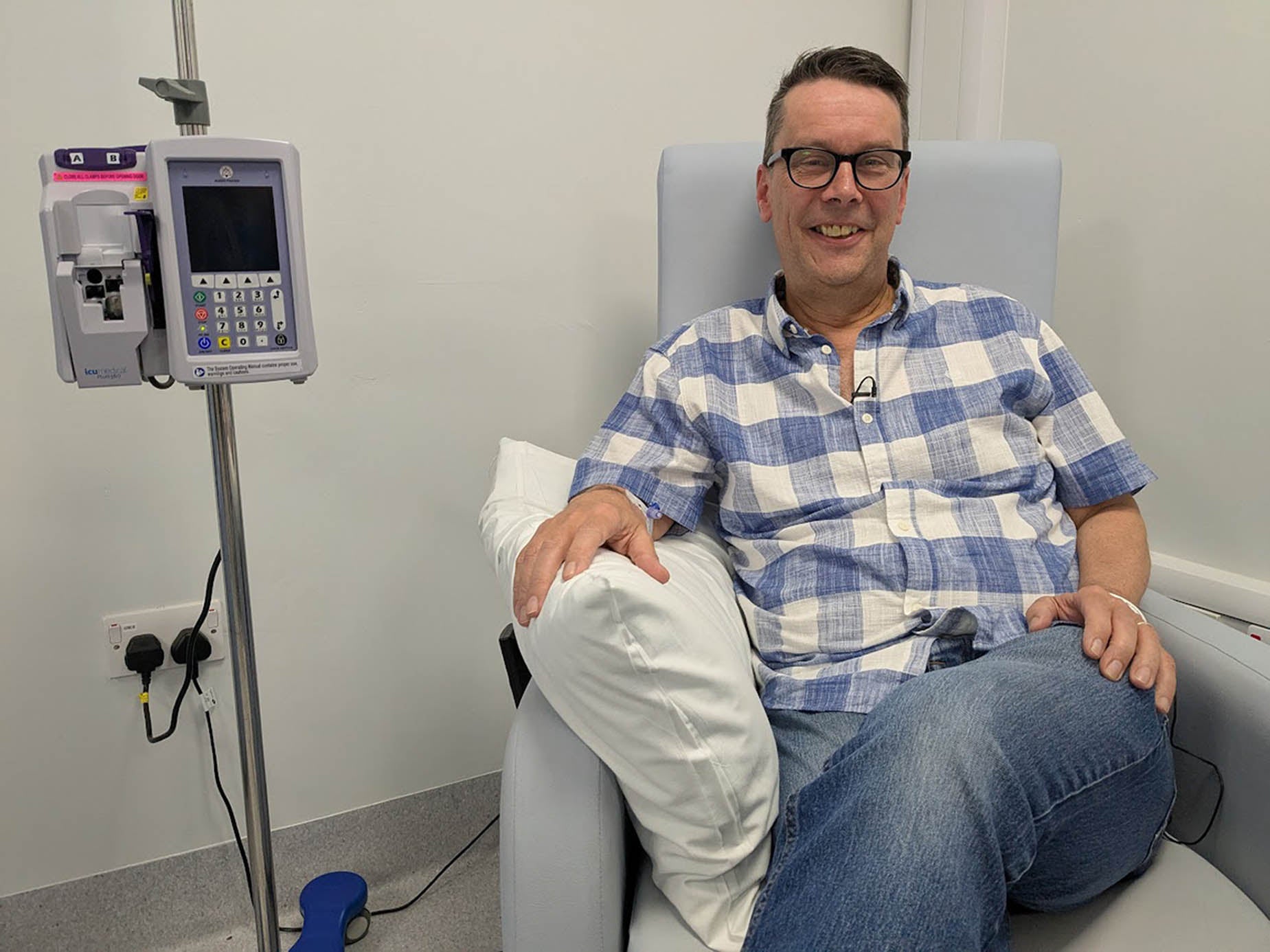
Patient Paul Silvester, 60, from Sheffield, was diagnosed with myeloma in July 2023 and received treatment at the Royal Hallamshire Hospital.
The first treatment he was given failed to stop his cancer from progressing so he was given belantamab mafodotin through an early access programme.
“I feel like this treatment has brought the party balloons back in the house. It has been amazing – within the first two or three weeks, after the first dose, I was in remission,” he said.
“It gives me quite a lot of confidence in the drugs and it makes me more optimistic about the future.
“I’ve been feeling well and I’m still quite active – that’s what’s important in terms of your quality of life.
“One of my daughters is graduating from university in October and it’s a goal for me to be there.”
Shelagh McKinlay, director of research and advocacy at blood cancer charity Myeloma UK, said: “It’s fantastic to see the UK at the forefront of myeloma treatment.
“We have been working very hard for the last year to get this treatment approved and we know it will transform the lives of thousands of people with myeloma.”
Health Minister Karin Smyth said: “This groundbreaking therapy puts the NHS at the forefront of cancer innovation.
“By harnessing cutting-edge ‘trojan horse’ technology, we’re offering new hope to blood cancer patients across the country.”




